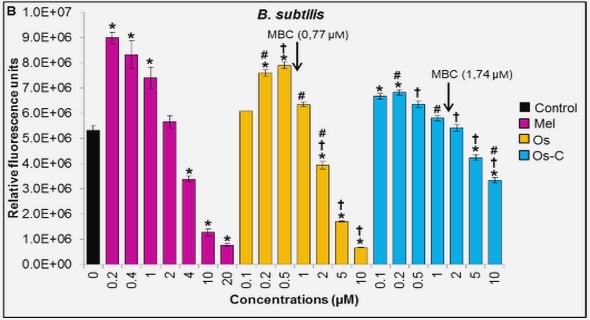
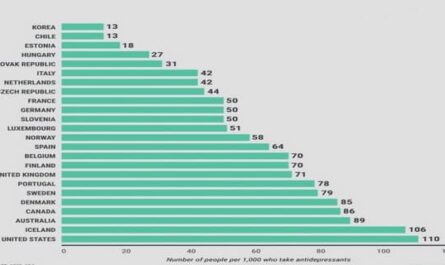
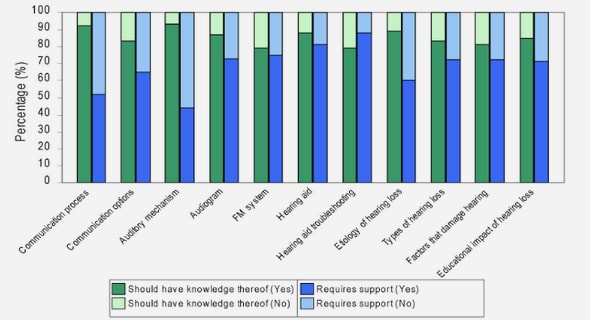
Get Complete Project Material File(s) Now! »
The serotonin transporter : cellular target of SSRIs during development
5-HTT is the primary target of the selective serotonin reuptake inhibitors (SSRIs). Therefore, identifying the cell types that expresses this transporter (SLC6A4) during development is vital to our understanding of the neurodevelopmental consequences of early SSRI exposure. In the adult brain, 5HTT expression is limited to the 5-HT producing neurons of the raphe nuclei. However, a much broader expression was observed in developing rodents (Gaspar et al., 2003; Narboux-Neme et al., 2008). Using genetic fate mapping approaches, our lab previously described a precise cellular localization of 5-HTT expression in the developing brain (Narboux-Neme et al., 2008). This made it possible to identify the principal targets of SSRIs during different phases of brain development. At mid gestation (embryonic day 11, E11, in mice) expression of the 5HTT gene begins in the 5-HT neurons of the raphe nuclei, but expression soon extends to non-serotonergic neurons, including the principal projection neurons of the sensory systems (thalamus, retina, somatosensory cortex), the corticolimbic pathways (hippocampus, E14– E15, and the prefrontal/cingulate cortex, postnatal day 0, P0). 5-HTT expression in these glutamatergic developing neurons ends rapidly during the second postnatal week, coinciding with the maturation of their neural circuits. Although studies in humans are limited, they do provide further evidence for the broad developmental expression of 5-HTT. In 8–11 gestational week-old embryos, 5-HTT immunolabelling was visible in fiber tracts of the internal capsule and in the optic tract. These fibers do not correspond to raphe projections (Verney et al., 2002). In new world monkeys (marmosets), 5-HTT was observed in all major sensory afferents (dorsal root ganglia, retinal ganglion cells, cochlear nucleus and olfactory nerves) at mid-gestation (Lebrand et al., 2006), an epoch which generally corresponds to the rodent perinatal period of neural circuit development. Unfortunately, the scarcity of biological material from primates has prevented evaluation of the precise time course of developmental 5-HTT expression, making it difficult to correlate these observations with the larger body of information collected from rodents (Homberg et al., 2010).
3.0 5-HT receptors
Studies dating back to key criteria for receptor characterization highlighted three main factors to consider. These include the operation or drug-related characteristics, signal transduction or receptor-effect coupling and the structural or gene and receptor structural sequences for their nucleotide and amino acid components (Humphrey et al., 1993). With this in mind, initial studies identified three 5-HT receptor families, 5-HT1-3 comprising five receptors/binding sites with the suspicion of some others (Bradley et al., 1986). At that time, a 5-HT autoreceptor function was ascribed to the 5-HT1-like receptor and it was hypothesized that the 5-HT3 receptor was mediating a depolarizing effect on the CNS neurons. However, not much was known about the functionality of the individual receptor subtypes (Barnes and Sharp, 1999).
The advent of more specific agonists and antagonists and in particular the advancement in molecular biology techniques allowed to identify 15 different 5-HT receptor subtypes, encoded by different genes in humans and rodents (Hoyer and Martin, 1997). It is now known that 5-HT, acts via two main categories of receptors – the ionotropic and metabotropic receptors. The ionotropic receptors or ligand-gated ion channels are identified by their low affinity for their neurotransmitter ligand but have a quick activation (in order of few milliseconds). In contrast, metabotropic receptors act via G-protein activation and second messenger production. They exhibit high ligand affinity and have a delayed activation, (in the order of seconds).
The only ionotropic 5-HT receptor is the 5-HT3 receptor. It has a pentameric structure. Functional channels may comprise of 5 identical 5-HT3a receptor subunits (homopentameric) or a combination of 5-HT3a and one of the other four subunits, 5-HT3b,c,d or e (heteropentameric). It seems that only the 5-HT3a receptor subunits is capable of forming functional homopentameric channels and all other subunit subtypes must heteropentamerize with 5-HT3a receptor subunits to form functional channels (Niesler et al., 2007). The 5-HT3 receptors are characterized by the occurrence of 4 transmembrane segments and a large extracellular N-terminal region. The functional receptor consists of 5 subunits arranged around a central ion conducting pore permeable to sodium, potassium and calcium ions. Upon activation by 5-HT the channel opens leading to an excitatory response in neurons.
All other 5-HT receptors are metabotropic. They are G-protein coupled receptors (GPCRs) having seven transmembrane domains. Based on their pharmacological properties, amino acid sequences, gene organization and second messenger coupling, the 15 receptor subtypes have been divided into six distinct classes – 5-HT1, 2, 4, 5, 6 and 7 receptors. The diversity of these receptors is further heightened by alternative splicing of some receptor subtypes.
Human association to depression phenotypes
Early studies on human subjects put forward a hypothesis of 5-HT deficiency in depression. This was proposed based on the findings of low 5-HIAA levels in the CSF of depressed patients (Sarna et al., 1983; Mignot et al., 1985) and suicidal victims (Asberg et al., 1986). These findings have been argued to reflect more the MAO activity than 5-HT release or utilization (Wolf et al., 1985). There have also been reports of low serotonergic activity inferred from allelic variation of genes involved in 5-HT synthesis, transmission and uptake in depressed individuals (Arango et al., 2003). In the late 90’s, Mayberg and colleagues proposed that depression involves a disruption of limbic-cortical pathways, based on evidence showing that regional glucose metabolic rate response (rCMRglu) is altered in depressed patients and that there are changes in brain metabolism after treatment with anti-depressants (Mayberg et al., 1997). This was further reinforced by the observation that depressed subjects exhibited significantly lower rCMRglu in the prefrontal and frontal cortices and in the inferior parietal lobe and the insula, compared to healthy subjects (Anderson et al., 2004).
Several 5-HT gene regulatory polymorphisms have been associated with negative emotionality and predisposition to depression and suicidality. In addition, stress hormones have been shown to be involved in regulating the expression of certain serotonin system genes. I will highlight a couple of the gene polymorphisms most linked to human emotional states.
The T allele is a functional polymorphism in the upstream regulatory region of TPH2 (SNP G-703T, rs4570625) which is responsible for low-expression of TPH2 (Gutknecht et al., 2007; Reuter et al., 2007). An impact of TPH2 variation on amygdala activation was reported by Milak and colleagues. Using positron emission tomography techniques on baboons, they found that transient reduction of cerebral 5-HT by acute tryptophan depletion increases amygdala’s responses to fearful faces. They argued that the increase amygdala response is likely due to a compensatory decrease of 5-HTT function and concomitant increase of synaptic 5-HT availability (Milak et al., 2005).
Another genetic polymorphism of 5-HT regulatory gene linked to major depression is the C(-1019)G 5-HT1A promoter polymorphism. Negative feedback inhibition of serotonergic raphe neurons is facilitated by somatodendritic 5-HT1A autoreceptors (Mongeau et al., 1997) and several antidepressant compounds desensitize raphe 5-HT1A autoreceptors consequently enhancing 5-HT neurotransmission (Albert et al., 1996; Artigas et al., 1996). Conversely, postmortem brains from depressed suicidal victims have shown increased 5-HT1A receptor densities in the raphe nuclei but not at postsynaptic sites. This translates to decreased serotonergic activity in depressed patients (Stockmeier et al., 1998). Lemonde et al., 2003 observed a two-fold increase in expression of the homozygous G(-1019) allele in depressed patients and a four-fold increase in expression of the same allele in suicide victims compared to controls. They proposed a model where the C(-1019)G polymorphism prevents binding of the transcriptional repressor – nuclear deformed epidermal autoregulatory factor (NUDR) therefore resulting in enhanced 5-HT1A receptor expression in raphe neurons (Lemonde et al., 2003).
In an association study describing the possible association of a T941G single nucleotide polymorphism (SNP) in the monoamine oxidase A (MAOA) gene with generalized anxiety disorder (GAD), panic disorder (PD), and major disorder (MD), Tadic and colleagues reported no association of the MAOA-T941G polymorphism with MD and PD but patients with GAD of Caucasian descent had significantly higher frequency of the 941T allele as compared to healthy subjects (Tadic et al., 2001). This SNP is located in the third base of a codon and does not affect the amino acid sequence. However, association of the allele with lower MAOA enzyme activity has been reported in cell lines of known MAOA activity (Hotamisligil and Breakefield, 1991). Supporting the notion of MAOA polymorphism and emotional disorders, a study conducted by Brunner in 1993 is arguably one of the clearest genetic evidence implicating MAOA activity in human behaviour. The authors identified a C to T point mutation at position 936 in the MAOA gene. This mutation introduced a premature termination codon leading to a nonfunctional MAOA protein. The males manifested borderline mental retardation, impulsivity, aggression, arson, attempted rape and exhibitionism (Brunner et al., 1993).
By regulating the degree and extent of serotonergic responses, the 5-HT transporter (5-HTT) is central to the fine-tuning of brain serotonergic neurotransmission and of the peripheral actions of 5-HT. The human 5HTT is encoded by a single gene (SLC6A4) on chromosome 17q12. 5HTT is the target of selective serotonin reuptake inhibitors (SSRIs) that block 5-HT reuptake with the effect of increasing the half-life of 5-HT at the synapses. The SLC6A4 gene promoter contains a glucocorticoid response element that makes it responsive to stress-induced levels of corticosteroids (Glatz et al., 2003; Lopez and Higley, 2002). Polymorphism in the translational control region upstream of the 5HTT coding sequence (5HTTLPR) – has been reported to give a long and short variant (the polymorphism consists of 44-base pair insertion or deletion) with different translational efficiencies (Lesch et al., 1996).
In the literature, the involvement of this 5HTTLPR polymorphism in emotional behaviours have been inconsistent and somewhat contradictory. There has been documented studies showing significant association between the low-expressing 5HTTLPR short variant and neuroticism with traits related to anxiety, stress reactivity and depression (Caspi et al., 2003). Since this discovery in 2003, many studies have investigated whether interaction of these polymorphisms with adverse environmental factors could be a predisposing factor to major depressive disorders. Using genome-wide association studies (GWAS), particularly gene x environment association, quite a number of research papers have argued that subjects with either one or two copies of the 5HTTLPR short variant had higher scores of neuroticism than those who were homozygous for the 5HTTLPR long variant (Canli and Lesch, 2007; Lesch et al., 1996; Xie et al., 2009; Grabe et al., 2012). Additionally, postmortem studies have described decreased 5HTT expression in brains of suicidal victims. However, the causal role of 5HTT polymorphism on depression is still debated. Some investigators did not find any correlation between depression risk and 5HTTLPR, whereas the effect of environmental factors such as lifetime and recent stressful events, sexual abuse, childhood trauma; on the prevalence and course of major depressive disorders was confirmed (Peyrot et al., 2013; Fisher et al., 2012). It should be noted that the model postulated by researchers in favour of a predisposition to negative emotional states by subjects carrying the s-allele of the 5HTTLPR is quite counter-intuitive considering antidepressants further inhibit the actions of 5HTT. As discussed later in experimental models, this argued for a developmental effect of changed 5HTT expression.
Animal model-based association to depression phenotypes
Studies looking at polymorphisms of 5-HT genes and association with depression phenotypes in nonhuman primates are few. A comparative study conducted by Lesch et al in 1997 described that variants of the 5HTTLPR are present only in simian primates (Lesch et al., 1997). They reported a long (419bp) and short (398bp) allelic variant of the 5HTTLPR in the rhesus monkey (rh-5HTTLPR). Because of their resemblance to humans in temperamental traits, Champoux and colleagues assessed the association between the rh-5HTTLPR and behavioural characteristics induced by 5-HT functioning in a sample of 115 infant rhesus monkeys with well characterized environmental histories. (Champoux et al., 2002) Their study showed 3 main observations – (1) a significant effect of genotype on emotionality – monkeys with the l/s genotype demonstrated more struggling, were less easily consoled, and manifested higher amounts of, and more frequent emotional distresses than the l/l homozygotic infants, (2) Nursery reared animals with the l/s genotype had substantially low orientation scores (an indirect measure of attention) compared to their maternally reared counterparts and (3) these observed behaviours became more obvious with increase in the age of the animals.
In a different experiment, the same authors assessed the effect of GxE in alcohol sensitivity. Here peer-reared (PR) and mother-reared (MR) monkeys with either the l/l or l/s genotype were infused with 16.8% ethanol through the saphenous veins and their intoxication scores were measured (Barr et al., 2003). They observed that animals homozygous for the l allele had significantly lower average intoxication scores compared to their l/s mates. Moreover, the PR animals with the l/l genotype had lower intoxication scores than did PR l/s animals. They concluded that, though their results cannot be directly translated to humans as quantifying alcohol intake is very complex, it is important to consider potential environmental influence on gene effects when studying the pathogenesis of alcohol dependence.
The rodent models support a significant role for serotonergic genotypes in determining psychiatric vulnerability. Investigating the effects of GxE as it relates to depressive phenotypes in rodents is only possible by the use of transgenic mouse or rat models. The down side of such models are the difficulty to really access the functional implication of the gene during developmental time point considering the life-long perturbation of the gene in question, leaving room for compensatory mechanisms. Therefore, using pharmacological tools (agonists and antagonists), the developmental function of a gene can be assessed during sensitive developmental windows.
In the rest of this chapter, while focusing on rats and mouse models, I will review concurrently, the effects of transgenic and pharmacological models (i.e. genetic inactivation of the gene and inactivation/activation during development respectively) of some 5-HT genes involved in depressive phenotypes within the scope of my thesis.
MAOA knockout mice were generated by the insertion of an interferon b (IFN-b) transgene between exons 1-4 of the MAOA gene. (Cases et al., 1995) the observed behaviours of pups were mostly locomotor, motor co-ordination defects. Similar to what had been observed in human males with a null MAOA mutation, adult mice showed increased aggressiveness, even among littermates. They had an anti-depressive phenotype when tested in the forced swim test, decreased anxiety as noted by longer time spent in the centre of the open field. At PND7, the authors observed disorganization of the barrel cortex, increased density of 5-HT staining by immunochemistry in the striatum, cortex, hilus of dentate gyrus and in neurons of the locus coeruleus and nigral complex, indicating an increase uptake of 5-HT (Cases et al., 1995, Vitalis et al., 1998). However, adult mice showed normal brain distribution and density. A reason for this could be the presence of the MAOB isoform present in non-neuronal cells in the adults (Cases et al., 1995; Vitalis et al., 1998). There is a gradual rise of MAOB specifically in postnatal life which could normalize adult monoamine metabolism (Tsang et al., 1986).
While MAOA action influences all monoamine levels, 5HTT function particularly regulates 5-HT signaling. Following the observation in humans that anti-depressants alleviates depressive symptoms, it was thought that generating a mouse model lacking 5HTT would be a good model to test the mechanisms underlying this. However, the contrary was observed because 5HTT knockout mice, had increased anxiety, learned helplessness, behavioural despair, and impaired social interaction (Ansorge et al., 2004; Kalueff et al., 2007; Lira et al., 2003; Muller et al., 2011). The 5-HTT knockout mice were generated by homologous recombination, by replacing the second exon of the SLC6A4 gene with a neomycin cassette (Bengal et al., 1998). This led to loss of the full length, functional 5HTT protein in the brain, and as expected, mice homozygous for the null mutation exhibit an absence of 5-HT reuptake, giving rise to a decreased rate of synaptic 5-HT clearance and a four- to six-fold increase in basal levels of forebrain extracellular 5-HT (Montanez et al., 2003).
Mutant mice lacking one SLC6A4 allele (5-HTT heterozygous) show a deficiency in 5-HT reuptake and clearance that is about 50% lower than controls (wildtype) (Bengal et al., 1998; Montanez et al., 2003). They reported only modest compensatory alterations in dopamine or norepinephrine function in 5HTT knockouts (Murphy et al., 2001; Montanez et al., 2003). There were, however, marked changes within the serotonergic system itself. 5HTT knockout mice showed a reduction in dorsal raphe neuronal firing and a desensitization and downregulation of somato-dendritic 5-HT1A autoreceptors (Gobbi et al., 2001; Li et al., 2000; Mannoury la Cour et al., 2001).
The 5HTT knockout mice showed reductions in the binding density of postsynaptic 5-HT1A receptors (125p-MPPI – selective radioligand of 5-HT1A) in the frontal cortex, amygdala, septum, and hypothalamus, but not the hippocampus (Li et al., 2000; Mannoury la Cour et al., 2001). Changes in the expression of other 5-HT receptor subtypes appeared to be less profound and more region-specific (Fabre et al., 2000).
Intracellular signaling of 5-HT7
Activation of the 5-HT7 receptor with 5-HT agonists is known to either activate the GS or Ga12 superfamily of G-proteins. The coupling of 5-HT7/Gs results in increased adenylyl cyclase (AC) activity which leads to the production of cAMP, which in turn activates protein kinase A (PKA) thereby promoting the phosphorylation of different downstream target proteins. cAMP production can also be regulated independently by intracellular Ca2+. This coupling of Ca2+ and cAMP has been shown to be one of several mechanisms underlying synaptic plasticity (Nielsen et al., 1996).
Table of contents :
INTRODUCTION
1.0 Development of emotional neural circuits
1,1 Critical periods in development
1.2 Critical periods in Limbic system development
2.0 Developmental role of Serotonin (5-HT)
2.1 Development of central 5-HT systems
2.2 The serotonin transporter: cellular target of SSRIs during development
3.0 5-HT receptors
4.0 5-HT regulated emotional and cognitive behaviours
4.1 Human association to depression phenotypes
4.2 Animal model-based association to depression phenotypes
4.3 5-HT regulation of PFC circuits
5 The 5-HT7 Receptor
5.1 Gene structure and alternative splicing
5.2 Intracellular signaling of 5-HT7
5.3 Tissue localization of the 5-HTR7 in the brain
5.4 Effect of 5-HT7 signalling on neuronal morphology and functions
5.5 Effects of 5-HT7 on other biological processes
CHAPTER I: Early life stress impairs postnatal oligodendendrogenesis and adult behavior through activity dependent mechanisms
CHAPTER II: SSRIs target prefrontal to raphe circuits during development modulating synaptic connectivity and emotional behavior
CHAPTER III: Serotonin and emotional behaviours: developmental role of the 5-HT7 receptor Materials and Methods
Animals
Brain tissue processing
Western Blot
Cell Cultures
RT-qPCRs
Drug Administration (5-HT7 receptor agonist and antagonist)
Viral injections in the PFC
Array tomography
Behavioural studies
Statistical analyses
RESULTS
The 5-HT7 receptor (Htr7) is expressed in the PFC during early postnatal development
Pharmacological blockade of 5-HT7 receptor during the postnatal periods interacts with developmental fluoxetine effects.
Emotional phenotype of the Htr7-KO mice; effects of PN-FLX in Htr7-/- and Htr7+/- mice
Overexpressing Htr7 in the developing PFC causes anxiety- and depressive-like behaviours in adulthood
Activation of PFC 5-HTR7 regulates PFC-DRN circuitry
DISCUSSION
Localization of Htr7 receptor in the mouse PFC during development
Pharmacological manipulation of 5-HT7 during development alleviates anxiety- and depressive-like behaviours caused by postnatal SSRI administration
Anatomical effects of 5-HT7 receptor stimulation
Future perspectives
REFERENCES