Get Complete Project Material File(s) Now! »
Removal of pharmaceutical micropollutants in MBRs
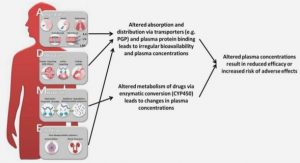
In the last decade, great attention has been paid to some emerging trace organic contaminants, also called micropollutants, such as endocrine-disrupting compounds, pharmaceuticals, personal care products etc., found in aquatic environment [47-48]. Among these micropollutants, pharmaceutical micropollutants got concerns since the late 1990s [49-51], as pharmaceuticals are designed to have some biological effects on living organisms and may have potential adverse effects on human health or aquatic organisms [52]. Also, concerns have been raised regarding that continuous discharge of antibiotics to aquatic environment may facilitate the development or proliferation of resistant strains of bacteria [53]. Moreover, chronic toxicity effects have been reported for aquatic organisms exposed to human pharmaceuticals at trace concentration [54-56]. So far, there is evidence of occurrence of some 160 different drugs in the effluents of wastewater treatment plants (WWTPs), and also of plants for the production of potable water from surface waters and groundwaters [57-58]. Pharmaceuticals found in water resources are classified into different therapeutic categories: analgesics and anti-inflammatory drugs, lipid regulators, antiepileptic drugs, beta-blockers, antibiotics, cytostatic drugs etc. Many pharmaceutical micropollutants, such as analgesics like ibuprofen, diclofenac, naproxen and ketoprofen, lipid regulators like bezafibrate and gemfibrozil, the antiepileptic drug carbamazepine, and antibiotics such as trimethoprim and sulfamethoxazole, have been frequently detected at concentration up to or higher than 1.0 µg/L in effluents of WWTPs around the world [59]. Pharmaceutical residues are discharged to sewers through a mixture of urine and faeces as the initial molecules or their metabolites after absorption by human bodies. Thus, pharmaceuticals mainly enter WWTPs via sewer networks after human release. Due to the incomplete removal or degradation of pharmaceutical micropollutants in conventional WWTPs, effluents discharged from WWTPs become a major source of pharmaceutical micropollutants entering into the water surface or underground resources [60-61].
Extensive studies have been carried out to investigate the removal efficiency and removal mechanisms of pharmaceutical micropollutants in MBR process, due to its potential advantages (such as higher SRT, accumulation of enzymatic biopolymers in the biological reactor) on removal of persistent organic pollutants as compared to the activated sludge process employed in conventional WWTPs. MBR was found to significantly enhance the removal of some partial degradable pharmaceutical micropollutants such as diclofenac, mefenamic acid, ketoprofen, clofibric acid, gemfibrozil etc. as compared to conventional activated sludge process (CAS) [62-64]. According to their removal efficiency in MBRs reported in the literature, pharmaceutical micropollutants can be classified into different groups of molecules [59], depending on their propensity to be removed in MBRs: (i) easily, with an average removal efficiency higher than 90 %, such as ibuprofen and bezafibrate; (ii) moderately, with an average removal efficiency of 50%-80%, such as naproxen, ketoprofen, gemfibrozil, trimethoprim, and sulfamethoxazole; (iii) poorly, with an average removal efficiency lower than 40%, such as diclofenac and carbamazepine. Thus, most pharmaceutical micropollutants are moderately removed in MBRs. In particular, carbamazepine is removed with an average removal efficiency of only 7% in MBRs [59].
The effects of pharmaceutical micropollutants on MBR process
Only a few studies examined the effects of the presence of pharmaceutical micropollutants on the MBR process, the majority of which focused on the effects on sludge microbial activity or the evolution of microbial community in the sludge. In batch experiments that used the activated sludge sampled from an MBR, Aubenneau et al. [76] examined the effect of trace concentration of carbamazepine on the mixed microbial communities and observed an inhibition of the exogenous respiration rate, a higher endogenous respiration rate, and a smaller floc size in presence of 1 µg L-1 carbamazepine in the sludge. Delgado et al. [77] reported that the continuous introduction of 5 µg L-1 of the cytostatic drug cyclophosphamide and its principal metabolites (CPs) in a MBR caused the inhibition of the exogenous respiration rate and increased the mean floc size of the activated sludge. Through the denaturing gradient gel electrophoresis (DGGE) analyses of the sludge community, Lay et al.
[23] observed shifts in microbial community and a reduced microbial diversity in the activated sludge several days after adding a mixture of carbamazepine, diclofenac, ibuprofen and naproxen in the feed tank (20–25 µg L-1 of each pharmaceutical in the feed) of an Osmotic Membrane Bioreactor (OMBR). Through the terminal restriction fragment length polymorphism (T-RFLP) analyses of the bacterial 16S rRNA genes, Kraigher et al. [78] observed shifts in the bacterial community structure in the activated sludge of a bioreactor continuously fed with a synthetic wastewater containing pharmaceuticals (ibuprofen, naproxen, ketoprofen, diclofenac and clofibric acid) at a concentration of 50 μgL−1, as compared to a control reactor, which was operated without addition of pharmaceuticals.
It is known that a chemical stress can induce the activated sludge to produce extracellular polymeric substances (EPSs) and soluble microbial products (SMPs), which are often identified as the main responsible for MBR fouling. The release of EPSs or SMPs may result from (i) the defensive response of bacteria to form a protective layer or (ii) decay (lysis) of bacteria and release of EPSs or SMPs inside the bacterial cells [79-80]. Still, very few studies reported the effects of pharmaceutical micropollutants on SMP production. In the literature, the effects of chemical stress on SMP production were examined in two ways:
(i) Long-term continuous contact. Lesage et al. [81] reported that after continuous addition of a toxic molecule, 2,4 dimethylphenol (DMP) in an MBR feed (90 mg/L in the feed), a significant increase in both protein and humic concentrations was observed. In contrast, the authors found no significant effect on polysaccharide concentration. Zhou et al.
[20] reported that an MBR fed with a municipal wastewater containing 0.4 mg L−1 Cr (VI) had a higher concentration of SMPs in the supernatant, especially in proteins compared with the MBR fed with the same municipal wastewater without addition of Cr (VI). Lay et al. [23] observed a significant increase in the protein and polysaccharide proportions for both soluble and bound EPS in the activated sludge after adding carbamazepine, diclofenac, ibuprofen and naproxen in the feed tank (20–25 µg L-1 of each pharmaceutical in the feed) of an Osmotic Membrane Bioreactor (OMBR).
(ii) Short-term discontinuous contact. Wang et al. [22] reported that a high osmotic pressure (5% NaCl) induces the production of SMPs with a relative strong hydrophilicity and protein-like characteristics in batch experiments. Sheng et al. [19] reported that in the presence of toxic chemicals, such as 30 mg l−1 Cu (II), 40 mg l−1 Cr (VI), 5 mg l−1 Cd (II) for a suspension of the bacteria strains Rhodopseudomonas acidophila, the EPS content increased by 5.5, 2.5, and 4.0 times, respectively. Moreover, the authors reported that under these toxic conditions, the increase in the protein content far exceeded the one of the other EPS constituents.
Physico-chemical properties of selected pharmaceutical micropollutant
Table 2 introduces the physicochemical properties of carbamazepine. During short-term batch experiments, a concentrated mother solution was prepared by dissolving carbamazepine (from Sigma-Aldrich) in pure ethanol at a concentration of 1 g/L, stored at 4 °C and used within a week. During long-term experiments, a concentrated mother solution was prepared by dissolving carbamazepine in pure ethanol at a high concentration of 10 g/L to rule out the effect of ethanol on the organic content in the feed. Then, quantities of this concentrated mother solution sufficient to yield about 90 µg L-1 final concentrations of CBZ were added in the feed tank of the MBR in step 2 after the steady state of the MBR reached, i.e. in 84th day, which is higher than 2 SRTs (30 days). The final concentration of ethanol in the feed was less than 0.001% (v/v).
Filterability tests
Fouling propensity of sludge and supernatant sampled from batch reactors (reactor-with CBZ and control-reactor) was characterized by filterability test performed with a stirred dead-end filtration cell (Amicon 8050, Millipore). Fig. 5 shows the schematic diagram of the filterability test. In the study of short-term CBZ effect, microfiltration membranes (from Alfa Laval, FR), made of polysulphone (PS), with a pore size of 0.2 μm (Lp0 = 200 l h-1 m-2 bar-1 at 20 °C), were used for the filterability tests of sludge, because a pore size of 0.2 μm is in the range of pore size of microfiltration membranes used in the flat sheet MBRs. Whereas, ultrafiltration membranes (from Orelis, FR), made of polyethersulfone (PES), with a nominal pore size of 0.01 μm (Lp0 = 50-80 L h-1 m-2 bar-1 at 20 °C) were used for the filterability tests of supernatant to retain biopolymer components in the MBR supernatant, behaving like the second layer on MBR membrane surface. A new membrane sample was used for each filtration test. All membranes were soaked in ultra pure water over night to maintain pores wetted. Then they were rinsed by filtration of ultra pure water at 1 bar during 15 min prior to filtration test. All filtration tests were carried out in an Amicon cell (Millipore), with an effective membrane filtration surface of about 13.4 cm2. The filtration tests were performed at three constant transmembrane pressures (TMP): 0.5 bar, 0.8 bar, and 1.0 bar. For each filtration test, sludge or supernatant was filtered through selected membranes until 70 ml permeate was produced (corresponding to a filtered permeate volume of about 50 L m-2); temperature was measured and flux was normalized to the flux at 20 °C. For supernatant filtration, no stirring was performed, and for sludge filtration, the sludge was stirred at 200 rpm. After filtration the membrane was turned face down and was backwashed with ultra pure water at 1 bar for 15 min, in order to investigate flux recovery by permeability measurement. In the study of long-term CBZ effect, ultrafiltration membranes (Microdyn-Nadir GmbH, Germany), made of polyethersulfone (PES), with a molecular weight cutoff (MWCO) 150 kDa (Lp0 = 400-600 L h-1 m-2 bar-1 at 20 °C) were used for the filterability tests of both sludge and supernatant. For the 150 kDa PES membranes, the same protocol for the filterability test was used except that prior to filtration test, they were rinsed by filtration of ultra pure water at 0.7 bar during 15 min and all filtration tests were carried out only at constant TMP of 0.5 bar. Membrane fouling resistance was determined according to Eq. (1). J TMP ( R R ) (1) m f.
Table of contents :
Publication 1. Evaluation of membrane bioreactor on removal of pharmaceutical micropollutants: a review
Publication 2. Effects of carbamazepine in peak injection on fouling propensity of activated sludge from a MBR treating municipal wastewater
Publication 3. Effects of pharmaceutical micropollutants on the membrane fouling of a submerged MBR treating municipal wastewater: case of continuous pollution by carbamazepine
Publication 4. HPLC-SEC with fluorescence detection as a tool for estimating fouling of MBRs used for municipal wastewater treatment
Résumé long en français